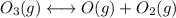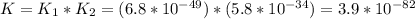Consider the following data. no2(g) equilibrium reaction arrow identifying the requirement for light no(g) + o(g) k = 6.8 ✕ 10-49 o3(g) + no(g) equilibrium reaction arrow no2(g) + o2(g) k = 5.8 ✕ 10-34 calculate a value for the equilibrium constant for the reaction below. (hint: when reactions are added together, the equilibrium expressions are multiplied.)

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:30, rosieposie27
(1.6 × 10-19)(5.0 × 106) = c × 10d identify the missing numbers below to show the result of multiplying the numbers.
Answers: 1


Chemistry, 22.06.2019 18:10, ellemarshall13
Measurements that have similar values are: a. usually accurate b. sometimes accurate c. always accurate d. never accurate
Answers: 1

Do you know the correct answer?
Consider the following data. no2(g) equilibrium reaction arrow identifying the requirement for light...
Questions in other subjects:


English, 31.08.2021 07:30


Mathematics, 31.08.2021 07:30

Mathematics, 31.08.2021 07:30

Geography, 31.08.2021 07:30


Biology, 31.08.2021 07:30

English, 31.08.2021 07:30


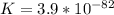
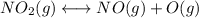
![K_1=\frac{[NO][O]}{[NO_2]}=6.8*10^{-49}](/tpl/images/0455/4423/c0bdd.png)

![K_2=\frac{[NO_2}{[O_3][NO]}=5.8*10^{-34}](/tpl/images/0455/4423/ae794.png)