Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 09:50, revlonknox6
Achemist has dissolved a certain substance in water. the chemist knows that more of the substance could be dissolved into the water before it stops dissolving. therefore
Answers: 2

Chemistry, 22.06.2019 12:30, gonzalesalexiaouv1bg
The missing component to the table to the right or indicated with orange letters complete the table by filling in the corresponding numbers or symbols
Answers: 3

Chemistry, 23.06.2019 00:30, evelynalper08
Which radioisotope is used to date fossils? a. oxygen-16 b. carbon-14 c. uranium-238 d. carbon-12
Answers: 2
Do you know the correct answer?
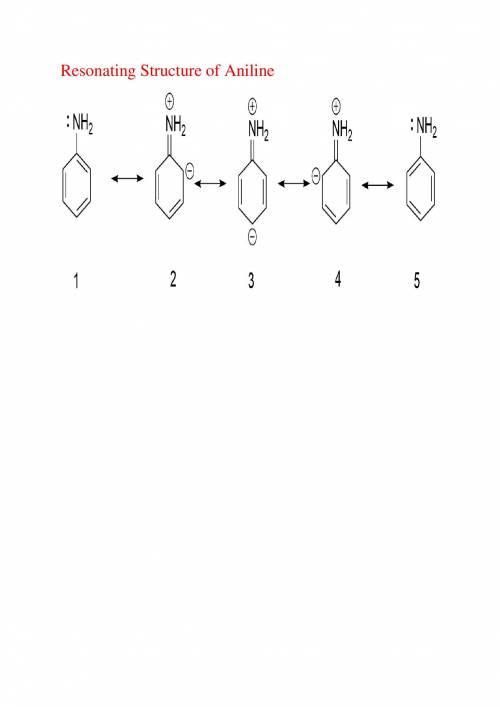
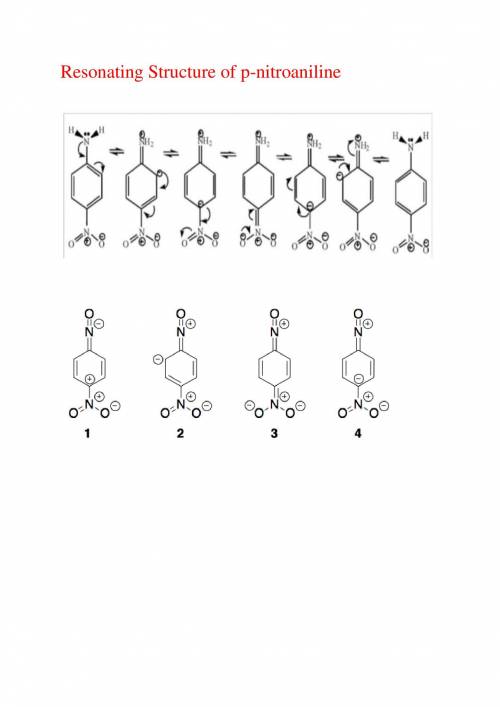
As the extent of electron delocalization into the ring increases, the geometry at nitrogen flattens....
Questions in other subjects:



Mathematics, 21.01.2021 23:00

Mathematics, 21.01.2021 23:00



History, 21.01.2021 23:00


Engineering, 21.01.2021 23:00