Chemistry, 14.01.2020 23:31, coochieboi
The gas arsine, ash3, decomposes as follows. 2 ash3(g) equilibrium reaction arrow 2 as(s) + 3 h2(g) in an experiment at a certain temperature, pure ash3(g) was placed in an empty, rigid, sealed flask at a pressure of 392.0 torr. after 48 hours the pressure in the flask was observed to be constant at 488.0 torr. (a) calculate the equilibrium pressure of h2(g).

Answers: 2
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 05:30, jzjajsbdb8035
Which other elements contain the same number of outer electrons as sodium
Answers: 3

Do you know the correct answer?
The gas arsine, ash3, decomposes as follows. 2 ash3(g) equilibrium reaction arrow 2 as(s) + 3 h2(g)...
Questions in other subjects:

History, 20.09.2020 05:01

Social Studies, 20.09.2020 05:01


Health, 20.09.2020 05:01

Mathematics, 20.09.2020 05:01






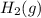 is, 288 torr
is, 288 torr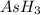 = 392.0 torr
= 392.0 torr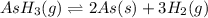
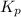 for the reaction will be:
for the reaction will be: