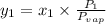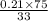Chemistry, 14.01.2020 20:31, danielamejia13
At 20 °c, the vapor pressure of benzene(c6h8) is 75 torr, and that of toluene(c7h8) is 22 torr. assuming that benzene and toluene from and idea solution.
a) what is the composition in mole fractions of a solution that has a vapor pressure of 33 torr at 20 °c
b) what is the mole fraction of benzene in the vapor above the solution described in part a?

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 21:30, leslyrivera11
If i make a solution by adding 83grams of sodium hydroxide to 750ml i’d water what is the molarity of sodium hydroxide
Answers: 1

Chemistry, 22.06.2019 13:30, kkingstone7062
What does the xylem do? stores the glucose captures the sunlight absorbs oxygen into the leaf carries water from the roots to the leaves
Answers: 1
Do you know the correct answer?
At 20 °c, the vapor pressure of benzene(c6h8) is 75 torr, and that of toluene(c7h8) is 22 torr. assu...
Questions in other subjects:

Mathematics, 06.01.2021 19:10

Mathematics, 06.01.2021 19:10

Mathematics, 06.01.2021 19:10

Mathematics, 06.01.2021 19:10


Mathematics, 06.01.2021 19:10

Mathematics, 06.01.2021 19:10

Mathematics, 06.01.2021 19:10

Mathematics, 06.01.2021 19:10


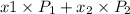
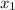 = mole fraction of component one
= mole fraction of component one
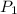 = vapor pressure of component one when pure
= vapor pressure of component one when pure
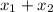 = 1
= 1
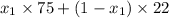
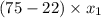
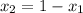
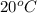 is 0.79.
is 0.79.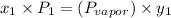
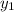 = composition in gas phase
= composition in gas phase