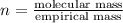Which statement is true about an empirical formula?
-it is the true ratio of atoms in a...

Chemistry, 26.10.2019 00:43, tfyvcu8052
Which statement is true about an empirical formula?
-it is the true ratio of atoms in a formula unit.
-it is the simplest ratio of atoms in a formula unit.
-it represents the true molecular mass of a chemical formula.
-it represents the highest ratio of coefficients in a chemical formula.

Answers: 3
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 10:30, ashlpiriz123
Find the number of grams of hcl needed to react completely with .50 moles of magnesium.
Answers: 1

Chemistry, 22.06.2019 11:30, charles8527
If blood contains 150g of hemoglobin per liter of blood, how much hemoglobin would be contained in 10 ml of blood
Answers: 2

Chemistry, 22.06.2019 22:00, huddyxo
Scientists often have to deal with numbers that are either very large or very small. for example, the radius of the sun is approximately 696,000 kilometers, while bacterial cells are as small as 1.9 × 10-4 millimeters. express each number in an alternate form.
Answers: 1
Do you know the correct answer?
Questions in other subjects:


Computers and Technology, 21.08.2019 03:20


Computers and Technology, 21.08.2019 03:20