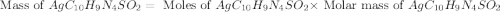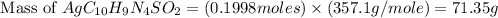Chemistry, 14.01.2020 01:31, xxaurorabluexx
Silver sulfadiazine burn-treating cream creates a barrier against bacterial invasion and releases antimicrobial agents directly into the wound. if 25.0 g of ag2o is reacted with 50.0 g of c10h10n4so2, what mass of silver sulfadiazine (agc10h9n4so2) can be produced, assuming 100% yield? ag2o(s)+2c10h10n4so2(s)⟶2agc10h9n4s o2(s)+h2o(l)

Answers: 3
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 08:30, MacenParisi
In the reaction between a crushed antacid tablet and vinegar what gas is emitted
Answers: 2


Chemistry, 23.06.2019 03:30, uniqueray33
The molar mass of nickel(ni) is 58.7 g/mol. how many moles are in an 88 gram sample of nickel?
Answers: 1
Do you know the correct answer?
Silver sulfadiazine burn-treating cream creates a barrier against bacterial invasion and releases an...
Questions in other subjects:










Computers and Technology, 07.03.2020 04:33


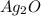 = 25.0 g
= 25.0 g = 50.0 g
= 50.0 g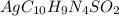 = 357.1 g/mole
= 357.1 g/mole
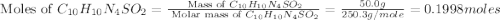

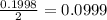 moles of
moles of