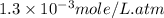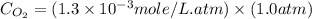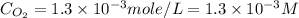Chemistry, 13.01.2020 21:31, aneisha0117
What is the molar concentration of oxygen in water at 25°c for a partial pressure of 1.0 atm ( = 1.3 x 10⁻³ mol/latm) a. 7.2 x 10⁻⁴b. 1.3 x 10⁻³ m c. 7.7 x 10⁴d. 1.3 x 10⁻⁵
= 1.3 x 10⁻³ mol/latm) a. 7.2 x 10⁻⁴b. 1.3 x 10⁻³ m c. 7.7 x 10⁴d. 1.3 x 10⁻⁵

Answers: 2
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 06:30, cadenhuggins2
Predict whether the changes in enthalpy, entropy, and free energy will be positive or negative for the boiling of water, and explain your predictions. how does temperature affect the spontaneity of this process?
Answers: 1

Chemistry, 22.06.2019 08:30, Apple557
The characteristic of two different types of reactions are shown below. reaction a: electrons are gained by the atoms of an element. reaction b: protons are lost by the atom of and element. which statement is true about the atoms of the elements that participate in the two reactions? a: their identity changes in both reaction a and b. b: their identity changes in reaction a but not b. c: their identity changes in reaction b but not a. d: their identity remains the same.
Answers: 1

Chemistry, 22.06.2019 18:30, TaraC
Read the claim. breakfast is an important meal. it jump starts the body’s process of using calories to break down food. appetite can decrease with age, but going too long without eating causes metabolism to slow down. current research shows that incorporating legumes such as lentils and chickpeas into meals boosts metabolism for twenty-four hours. who might benefit from this claim? people who have a fast metabolism stores that sell exercise equipment people who take vitamin supplements grocery stores that sell legumes
Answers: 1
Do you know the correct answer?
What is the molar concentration of oxygen in water at 25°c for a partial pressure of 1.0 atm ([tex]k...
Questions in other subjects:



History, 31.08.2019 08:30


Mathematics, 31.08.2019 08:30

Social Studies, 31.08.2019 08:30

Mathematics, 31.08.2019 08:30



Biology, 31.08.2019 08:30


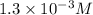
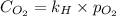
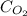 = molar concentration of
= molar concentration of 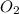 = ?
= ?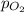 = partial pressure of
= partial pressure of 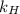 = Henry's law constant =
= Henry's law constant =