Given 24.3 g of lici, find moles of pbci, produced.
pbso4 + licl → pbcl2 + li2so4
...

Chemistry, 13.01.2020 07:31, jodygoodwin40
Given 24.3 g of lici, find moles of pbci, produced.
pbso4 + licl → pbcl2 + li2so4
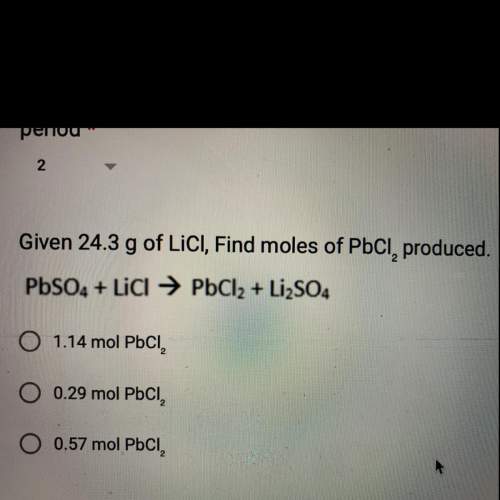

Answers: 3
Other questions on the subject: Chemistry


Chemistry, 23.06.2019 00:30, runninglovexoxo
The molecular weight of carbon dioxide, co2, is 44.00 amu, and the molecular weight of nitrous dioxide, no2, is 46.01 amu, so no2 diffuses co2
Answers: 2

Chemistry, 23.06.2019 01:40, mandilynn22
Calcium carbonate decomposes at high temperatures to give calcium oxide and carbon dioxide as shown below. caco3(s) cao(s) + co2(g) the kp for this reaction is 1.16 at 800°c. a 5.00 l vessel containing 10.0 g of caco3(s) was evacuated to remove the air, sealed, and then heated to 800°c. ignoring the volume occupied by the solid, what will be the mass of the solid in the vessel once equilibrium is reached?
Answers: 1

Do you know the correct answer?
Questions in other subjects:


Social Studies, 07.12.2021 17:50

Chemistry, 07.12.2021 17:50

English, 07.12.2021 17:50

Spanish, 07.12.2021 17:50

Mathematics, 07.12.2021 17:50

English, 07.12.2021 17:50


English, 07.12.2021 17:50






