
In an experiment, potassium chlorate decomposed according to the following chemical equation. kclo3 → kcl + o2 (molar mass of kclo3 = 122.5 g/mol; kcl = 74.55 g/mol; o2 = 31.998 g/mol) if the mass of kcl produced was 180 grams, which of the following calculations can be used to determine the mass of potassium chlorate decomposed? (180 × 2 × 74.55) ÷ (122.5 × 2) grams (180 × 3 × 74.55) ÷ (122.5 × 2) grams (180 × 2 × 122.5) ÷ (74.55 × 2) grams (180 × 3 × 122.5) ÷ (74.55 × 2) grams

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 01:00, bettybales1986
According to the tide table below what time of day will the highest tide occur?
Answers: 1

Chemistry, 22.06.2019 06:00, josmanu235
Why is permeable soil best for plants that need a lot of drainage?
Answers: 1


Chemistry, 23.06.2019 00:30, cashkidd2200
There are approximately 15 milliliters (ml) in 1 tablespoon (tbsp). what expression can be used to find the approximate number of milliliters in 3 tbsp?
Answers: 1
Do you know the correct answer?
In an experiment, potassium chlorate decomposed according to the following chemical equation. kclo3...
Questions in other subjects:



History, 02.10.2019 21:50

English, 02.10.2019 21:50

Geography, 02.10.2019 21:50



History, 02.10.2019 21:50

Mathematics, 02.10.2019 21:50

Chemistry, 02.10.2019 21:50


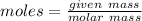
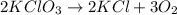
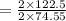 of KClO3
of KClO3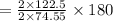 of KClO3
of KClO3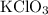 has been given by
has been given by 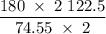 grams. Thus option C is correct.
grams. Thus option C is correct.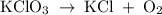
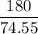 moles
moles molecular weight
molecular weight



