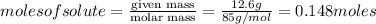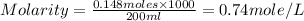Chemistry, 11.01.2020 05:31, tsunommygames
Show the calculation of the molarity of a solution made by dissolving 12.6 grams of nano3 to make 200 ml of solution.

Answers: 3
Other questions on the subject: Chemistry



Chemistry, 22.06.2019 18:00, meowmeowcow
Find the mass, in grams, of 5.00*10^23 molecules of f2
Answers: 3

Chemistry, 22.06.2019 18:30, chinadoll24
Asample of hydrated tin (ii) chloride (sncl2) has a mass of 4.90 g. when it is dehydrated, it has a mass of 4.10 g. which is the correct chemical formula for the hydrate? sncl2•2h2o sncl2•4h2o sncl2•6h2o
Answers: 2
Do you know the correct answer?
Show the calculation of the molarity of a solution made by dissolving 12.6 grams of nano3 to make 20...
Questions in other subjects:


History, 25.11.2019 16:31

Mathematics, 25.11.2019 16:31





Chemistry, 25.11.2019 16:31



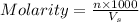
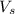 = volume of solution in ml = 1000 ml
= volume of solution in ml = 1000 ml