
Chemistry, 11.01.2020 03:31, eichlingkera3
3c2h2(g) → c6h6(g) what is the standard enthalphy change δho, for the reaction represented above? (δhof of c2h2(g) is 230 kj mol-1; (δhof of c6h6(g) is 83 kj mol-1; )

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 14:00, kelyanthecrafte
Which of the following best defines homeostasis? forming identical cells breaking down glucose maintaining stable internal conditions increasing an organism's temperature
Answers: 3

Chemistry, 21.06.2019 17:10, codeyhatch142
Nitric oxide (no) can be formed from nitrogen, hydrogen and oxygen in two steps. in the first step, nitrogen and hydrogen react to form ammonia: n2(g) + 2 h_2(g) rightarrow 2 nh_3 (g) delta h = -92. kj in the second step, ammonia and oxygen react to form nitric oxide and water: 4 nh_3(g) + 5 o_2(g) rightarrow 4no(g) + 6 h_2o(g) delta h = -905. kj calculate the net change in enthalpy for the formation of one mole of nitric oxide from nitrogen, hydrogen and oxygen from these reactions. round your answer to the nearest .
Answers: 1

Do you know the correct answer?
3c2h2(g) → c6h6(g) what is the standard enthalphy change δho, for the reaction represented above? (...
Questions in other subjects:

Chemistry, 02.10.2020 15:01


Mathematics, 02.10.2020 15:01

Law, 02.10.2020 15:01




Mathematics, 02.10.2020 15:01

Business, 02.10.2020 15:01


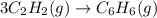
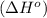 .
.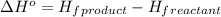
![\Delta H^o=[n_{C_6H_6}\times \Delta H_f^0_{(C_6H_6)}]-[n_{C_2H_2\times \Delta H_f^0_{(C_2H_2)}]](/tpl/images/0450/4494/c3760.png)
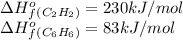
![\Delta H^o_{rxn}=[(1\times 83)]-[(3\times 230)]=-607kJ](/tpl/images/0450/4494/ed5f4.png)





