
Chemistry, 10.01.2020 06:31, aidanwindsor1738
Asource of zinc metal can be zinc ore containing zinc(ii) sulfide. the ore is roasted in pure oxygen to produce the oxide and then reduced with carbon to form elemental zinc and carbon monoxide. 2 zns + o2 2 zno + 2 so2 zno + c zn + co a crucible containing a sample of 0.50 mol zns was roasted in pure oxygen, then reduced with 1.00 mol carbon. what mass remained in the crucible after cooling?

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:30, rosetoheart2
What are the three major branches of natural science? • earth and space science, life science, physical science •earth and space science, physical science, chemistry •physical science, life science, chemistry •life science, chemistry, physics
Answers: 1

Chemistry, 22.06.2019 07:30, veronica25681
According to the vsepr theory what is the shape of a molecule that has a central atom valence three other items with no lone pairs of electrons
Answers: 1

Chemistry, 22.06.2019 14:50, chem1014
Given the following information: mass of proton = 1.00728 amu mass of neutron = 1.00866 amu mass of electron = 5.486 × 10^-4 amu speed of light = 2.9979 × 10^8 m/s calculate the nuclear binding energy (absolute value) of 3li^6. which has an atomic mass of 6.015126 amu. j/mol.
Answers: 2

Chemistry, 22.06.2019 16:00, hjgjlgkjg
Inside a flashbulb, oxygen surrounds a thin coil of magnesium. when the flashbulb is set off, a chemical reaction takes place in which magnesium combines with oxygen to form magnesium oxide. which of the chemical equations matches the reaction above? a. mg + o2 mgo2 + energy b. 2mg + o mg2o + energy c. 2mg + o2 2mgo + energy d. mg + o mgo + energy
Answers: 1
Do you know the correct answer?
Asource of zinc metal can be zinc ore containing zinc(ii) sulfide. the ore is roasted in pure oxygen...
Questions in other subjects:

Health, 28.11.2019 13:31


Mathematics, 28.11.2019 13:31







Health, 28.11.2019 13:31


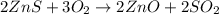 ..[1]
..[1]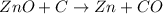 ..[2]
..[2]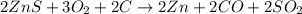 ..[3]
..[3]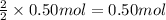 of Zn.
of Zn.



