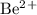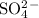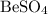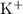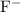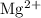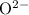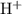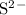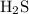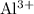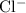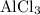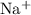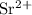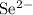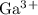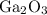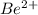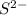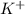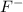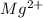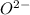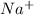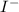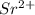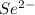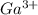Chemistry, 09.01.2020 10:31, shelovejaylocs
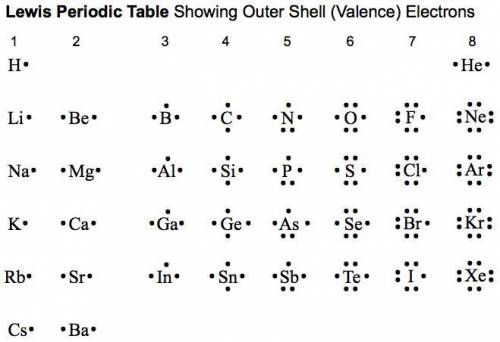
1. use electron dot formulas to predict the formulas of the ionic compounds formed from the
following elements:
a) beryllium and sulfur
b) potassium and fluorine
c) magnesium and oxygen
d) hydrogen and sulfur
e) aluminum and chlorine
f) iodine and sodium
g) selenium and strontium
h) gallium and oxygen

Answers: 2
Other questions on the subject: Chemistry



Chemistry, 22.06.2019 09:00, tashaunalewis4786
This chart lists four kinds of polymers and their sources. what can be known about all four polymers, despite their differences? they come from living things. they share ionic carbon bonds. they are at least 100 monomers long. they are made of repeating subunits.
Answers: 1

Chemistry, 22.06.2019 21:30, crystalbyrd79p8imrx
What is the effect of returning nuclear reactor cooling water back into bodies of water?
Answers: 3
Do you know the correct answer?
1. use electron dot formulas to predict the formulas of the ionic compounds formed from the
Questions in other subjects:

Chemistry, 13.01.2021 20:30


Mathematics, 13.01.2021 20:30

English, 13.01.2021 20:30

Mathematics, 13.01.2021 20:30

Physics, 13.01.2021 20:30


Chemistry, 13.01.2021 20:30

History, 13.01.2021 20:30

English, 13.01.2021 20:40