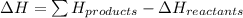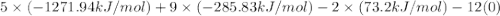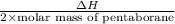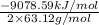Pentaborane-9,
, is a colorless, highly reactive liquid thatwill burst into flame
when exposed to oxygen. the reactionis:
calculate the kilojoules of heat released per gram of
thecompound reacted with oxygen. the standard enthalpy of formation
ofb5 h9 is 73.2 kj/mol.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:30, darrriannn7241
What is the correct lewis structure for chloroform chcl3
Answers: 1

Chemistry, 22.06.2019 22:30, lori90
Essay-alternative energy sources research sources of energy that are being developed. write a report of 350-400 words discussing the information you learned concerning the development of various energy sources and the impact that you think they will have on your life. include sources cited at the end of your report using the mla format. follow the rubric guidelines. note that wikipedia is not an appropriate resource for a research paper. worth 99
Answers: 3

Chemistry, 23.06.2019 06:30, fjsdfj1284
Moving force of air flows from areas of high pressure to areas of low pressure true or false
Answers: 2

Chemistry, 23.06.2019 09:20, goldwinner300
Asolution of naoh has a concentration of 25.00% by mass. what mass of naoh is present in 0.250 g of this solution? use the periodic table in the toolbar if needed. 0.0625 g what mass of naoh must be added to the solution to increase the concentration to 30.00% by mass? g
Answers: 2
Do you know the correct answer?
Pentaborane-9,
, is a colorless, highly reactive liquid thatwill burst into flame
when...
, is a colorless, highly reactive liquid thatwill burst into flame
when...
Questions in other subjects:

Mathematics, 05.12.2020 18:40



Chemistry, 05.12.2020 18:40

Arts, 05.12.2020 18:40

Mathematics, 05.12.2020 18:40

English, 05.12.2020 18:40

Mathematics, 05.12.2020 18:40



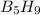 ) is a colorless highly reactive liquid that will burst into flames when exposed to oxygen.the reaction is:
) is a colorless highly reactive liquid that will burst into flames when exposed to oxygen.the reaction is:
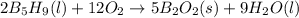
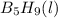 ,
, 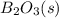 , and
, and 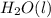 are 73.2, -1271.94, and -285.83 kJ/mol, respectively.
are 73.2, -1271.94, and -285.83 kJ/mol, respectively.