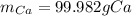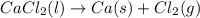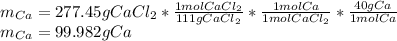Chemistry, 08.01.2020 12:31, sheldonwaid4278
Cacl2 can be melted to produce calcium metal and give off chlorine gas. the equation for this is cacl2(l) ca(s) + cl2(g). if 277.45 g cacl2 were melted, how many grams of ca(s) would be formed? (

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 17:30, kev71
You are performing an experiment in a lab to attempt a new method of producing pure elements from compounds. the only problem is that you do not know what element will form. by your previous calculations you know that you will have 6.3 moles of product. when it is complete, you weigh it and determine you have 604.4 grams. what element have you produced?
Answers: 1


Chemistry, 22.06.2019 06:30, cadenhuggins2
Predict whether the changes in enthalpy, entropy, and free energy will be positive or negative for the boiling of water, and explain your predictions. how does temperature affect the spontaneity of this process?
Answers: 1

Chemistry, 22.06.2019 13:30, richardwalker8ourhg2
An animal cell loses the ability to convert energy stored in food to energy that the cell can use. which of the cell's organelles has stopped working? a. the mitochondria b. the nucleus c. the vacuoles d. the endoplasmic reticulum
Answers: 1
Do you know the correct answer?
Cacl2 can be melted to produce calcium metal and give off chlorine gas. the equation for this is cac...
Questions in other subjects:

Mathematics, 07.04.2021 16:50




Mathematics, 07.04.2021 16:50



Mathematics, 07.04.2021 16:50

World Languages, 07.04.2021 16:50