1) given the balance equation below. calculate how much na3po4 in grams you
would need to creat...

Chemistry, 07.01.2020 19:31, dyanaycooper13
1) given the balance equation below. calculate how much na3po4 in grams you
would need to create 100 grams of naoh.
1 na3po4 + 3 koh → 3 naoh + 1 k3po4

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 13:50, 24lbriscoe
It has been two weeks since charles met with daniel, a dietitian, who provided charles with a menu for weight loss. charles and his mother are going back to see daniel again with a chart of the food charles has eaten. the following lists what charles ate in one day: breakfast 1 banana, 1 cup of nonfat milk, 1 egg lunch 1 cup of carrots, 3 oz of steak, 1 apple, 1 cup of nonfat milk dinner 6 oz of skinless chicken, 1 baked potato, 3 oz of broccoli, 1 cup of nonfat milk
Answers: 1


Chemistry, 22.06.2019 20:00, SpiritedAway7087
In vapor-liquid equilibrium in a binary mixture, both components are generally present in both phases. how many degrees of freedom are there for such a system? the reaction between nitrogen and hydrogen to form ammonia occurs in the gas phase. how many degrees of freedom are there for this system? steam and coal react at high temperatures to form hydrogen, carbon monoxide, carbon dioxide, and methane. the following reactions have been suggested as being involved in the chemical transformation:
Answers: 3

Chemistry, 22.06.2019 21:20, jordan2875
If a simple machine aduces the strength of a force, what must be increased? the speed of the input force the work the simple machine performs the size of the simple machine the distance over which the force is applied
Answers: 1
Do you know the correct answer?
Questions in other subjects:

Mathematics, 03.11.2020 22:00

Business, 03.11.2020 22:00

Mathematics, 03.11.2020 22:00

Mathematics, 03.11.2020 22:00


Mathematics, 03.11.2020 22:00


History, 03.11.2020 22:00

History, 03.11.2020 22:00

Spanish, 03.11.2020 22:00


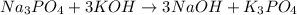
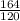
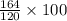 gram of Na3PO4
gram of Na3PO4



