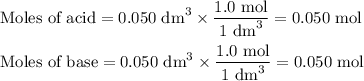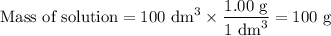Chemistry, 05.01.2020 17:31, gharrell03
50cm3 of 1 mol/dm3 hcl at 30°c was mixed with 50cm3 of 1mol/dm3 naoh at 30°c in a styrofoam calorimeter. the temperature of the calorimeter rose by 4.5°c. calculate the heat of reaction per mol of h20 formed.( heat capacity of the calorimeter is 50j/°c

Answers: 1
Other questions on the subject: Chemistry



Chemistry, 22.06.2019 16:10, 00015746
Predict the reactants of this chemical reaction. that is, fill in the left side of the chemical equation. be sure the equation you submit is balanced. (you can edit both sides of the equation to balance it, if you need to.) note: you are writing the molecular, and not the net ionic equation. > cacl2(aq) + h20(l)
Answers: 2

Chemistry, 23.06.2019 03:50, arimarieestrada
How many liters of oxygen gas, at standardtemperature and pressure, will react with 35.8 grams ofiron metal? 4 fe (s) + 3 o2 (g) → 2 fe2o3 (s)
Answers: 3
Do you know the correct answer?
50cm3 of 1 mol/dm3 hcl at 30°c was mixed with 50cm3 of 1mol/dm3 naoh at 30°c in a styrofoam calorime...
Questions in other subjects:





Social Studies, 15.04.2020 19:36




Mathematics, 15.04.2020 19:37

History, 15.04.2020 19:38