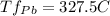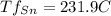Alead–tin alloy of composition 30 wt% sn–70 wt% pb is slowly heated from a temperature of 150°c (300°) at what temperature does the first liquid phase form? (b) what is the composition of this liquid phase? (c) at what temperature does complete melting of the alloy occur? (d) what is the composition of the last solid remaining prior to complete melting?

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 15:30, hannah5143
Determine the empirical formula of a compound containing 40.6 grams of carbon, 5.1 grams of hydrogen, and 54.2 grams of oxygen. in an experiment, the molar mass of the compound was determined to be 118.084 g/mol. what is the molecular formula of the compound? for both questions, show your work or explain how you determined the formulas by giving specific values used in calculations.
Answers: 3

Chemistry, 21.06.2019 20:30, Bradgarner772
18. use the activity series to predict whether the following synthesis reaction will occur. write the chemical equations for the reaction if it's predicted to occur. (s) + o2(g) -> *note: it is possible.*
Answers: 1

Chemistry, 22.06.2019 02:00, webbhlharryteach
In which of these cases are the two wave points considered to be in phase with each other?
Answers: 1

Chemistry, 22.06.2019 02:30, rileyeddins1010
List four observations that indicate that a chemical reaction may be taking place
Answers: 1
Do you know the correct answer?
Alead–tin alloy of composition 30 wt% sn–70 wt% pb is slowly heated from a temperature of 150°c (300...
Questions in other subjects:


Social Studies, 17.09.2019 13:50




History, 17.09.2019 13:50

Biology, 17.09.2019 13:50

Spanish, 17.09.2019 13:50

History, 17.09.2019 13:50