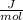Chemistry, 03.01.2020 03:31, lucywood2024
Carbon monoxide at 25°c and steam at 150°c are fed to a continuous water-gas shift reactor. the product gas, which contains 50.0 mole% h2, 40.0% , and the balance emerges at 500°c at a rate of 2.50m3/h and goes to a condenser. the gas and liquid streams leaving the condenser are in equilibrium at 15°c and 1 bar. the liquid may be taken to be pure w"a) calculate the % excess steam fed to the reactor and rate of condensation of the water (kg/h) leaving the condensor. b) calculate the rate (kw) at which heat must be removed from the condensor. c) calculate the rate of heat transfer (kw) to or from the reactor (state which it is).

Answers: 2
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 23:30, treylartigue
The appropriate concentration for an iodine sanitizer is
Answers: 1


Chemistry, 23.06.2019 03:00, jaidencoolman2866
Determine type of reaction & predict the product c3h12+o2 =
Answers: 1
Do you know the correct answer?
Carbon monoxide at 25°c and steam at 150°c are fed to a continuous water-gas shift reactor. the prod...
Questions in other subjects:









Chemistry, 29.04.2021 01:00


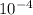 kW
kW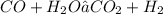
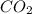 = 40 moles
= 40 moles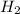 = 40 moles
= 40 moles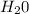 = 20 moles
= 20 moles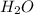
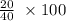 %
%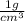
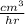
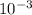
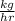
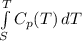
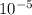 (-16324.231)
(-16324.231)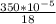 (-16324.231) *
(-16324.231) *