Chemistry, 03.01.2020 02:31, oliviaparker2010
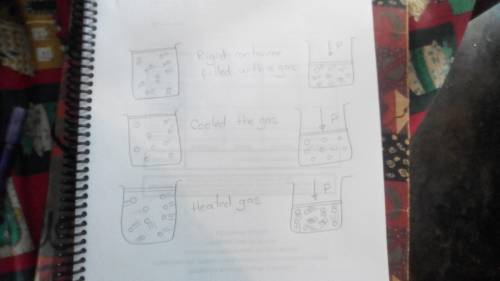
Draw a highly magnified view of a sealed, rigid container filled with a gas. then draw what it would look like if you cooled the gas significantly but kept the temperature above the boiling point of the substance in the container. also draw what it would look like if you heated the gas significantly. finally, draw what each situation would look like if you evacuated enough of the gas to decrease the pressure by a factor of 2.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:30, BLASIANNkidd
Maria wants to determine which type of disinfectant kills the most bacteria. which of the following is the best way for maria to determine this? a. ask ten different companies that make disinfectants which type is best. b. put the same amount and species of bacteria on ten identical plates, and add ten different kinds of disinfectant to each plate. c. interview ten different people to determine which type of disinfectant they prefer. d. put the same amount and species of bacteria on ten identical plates, and add a different disinfectant to each plate.
Answers: 1

Chemistry, 22.06.2019 01:30, EMQPWE
In a spacecraft, the following reaction occurs: co2(g) + 2lioh(s) -> lico3(s) + h2o(i) (i attached picture of equation) how many liters of carbon dioxide will 4 moles of lithium hydroxide (lioh) absorb? (one mole of any gads occupies 22.4 l under certain conditions of temperature and pressure. assume those conditions for this equation.) 45l 6.0l 3.0l 34l
Answers: 1

Chemistry, 22.06.2019 09:40, kolibeilfuss
Sulfur dioxide and oxygen react to form sulfur trioxide during one of the key steps in sulfuric acid synthesis. an industrial chemist studying this reaction fills a 25.0l tank with 4.5 mol of sulfur dioxide gas and 4.5 mol of oxygen gas at 30.°c. he then raises the temperature, and when the mixture has come to equilibrium measures the amount of sulfur trioxide gas to be 1.4 mol. calculate the concentration equilibrium constant for the reaction of sulfur dioxide and oxygen at the final temperature of the mixture. round your answer to 2 significant digits.
Answers: 3
Do you know the correct answer?
Draw a highly magnified view of a sealed, rigid container filled with a gas. then draw what it would...
Questions in other subjects:

Physics, 24.12.2019 03:31

Mathematics, 24.12.2019 03:31


Mathematics, 24.12.2019 03:31

Mathematics, 24.12.2019 03:31


History, 24.12.2019 03:31