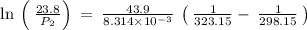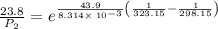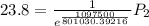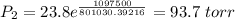Chemistry, 03.01.2020 02:31, jeffhuffle17
The vapor pressure of water at 25 degrees celsius is 23.8 torr, and the heat of vaporization of water at 25 degrees celsius is 43.9 kj/mol. calculate the vapor pressure of water at 50 degrees celsius.

Answers: 2
Other questions on the subject: Chemistry



Chemistry, 22.06.2019 07:30, gwenparks
Calculate the ratio of h+ ions to oh– ions at a ph = 7. find the concentration of h+ ions to oh– ions listed in table b of your student guide. then divide the h+ concentration by the oh– concentration. record this calculated ratio in table a of your student guide. compare your approximated and calculated ratios of h+ ions to oh– ions at a ph = 7. are they the same? why or why not? record your comparison in table a. what is the concentration of h+ ions at a ph = 7? mol/l what is the concentration of oh– ions at a ph = 7? mol/l what is the ratio of h+ ions to oh– ions at a ph = 7? : 1
Answers: 1

Chemistry, 22.06.2019 08:20, pilarmonsivais
What is the formula for the compound dinitrogen pentoxide? a. n4o5 b. n5o4 c. n4o6 d. n5o2 e. n2o5
Answers: 3
Do you know the correct answer?
The vapor pressure of water at 25 degrees celsius is 23.8 torr, and the heat of vaporization of wate...
Questions in other subjects:

Mathematics, 17.11.2020 07:50





History, 17.11.2020 07:50


Mathematics, 17.11.2020 07:50


English, 17.11.2020 07:50


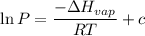
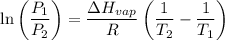
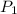 = 23.8 torr
= 23.8 torr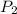 = ?
= ?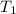 = 25°C
= 25°C
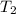 = 50 °C
= 50 °C