
Part d 2clo2(g)+2i−(aq)→2clo−2(aq)+i2(s) drag the appropriate labels to their respective targets. e−→e superscript- rightarrow ←e−leftarrow e superscript- cathode cathode anode anode ii superscript- clo−2c l o subscript 2 superscript- request answer part e indicate the half-reaction occurring at anode. express your answer as a chemical equation. identify all of the phases in your answer. nothing

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 03:30, electrofy456
What diagram shows the ionic compound of magnesium oxide
Answers: 2

Chemistry, 22.06.2019 04:50, psychocatgirl1
Write the overall equation for the reaction for lithium battery
Answers: 2

Chemistry, 22.06.2019 06:00, lindseyklewis1p56uvi
Ethanol (c2h5oh) is produced from the fermentation of sucrose in the presence of enzymes. c12h22o11(aq) + h2o(g) 4 c2h5oh(l) + 4 co2(g) determine the theoretical yield and the percent yields of ethanol if 680. g sucrose undergoes fermentation and 326.5 g ethanol is obtained. theoretical _ g _ percent %
Answers: 1

Chemistry, 22.06.2019 10:00, melissa9882
A50.0g sample of liquid water at 0.0 c ends up as ice at -20.0 c. how much energy is involved in this change?
Answers: 1
Do you know the correct answer?
Part d 2clo2(g)+2i−(aq)→2clo−2(aq)+i2(s) drag the appropriate labels to their respective targets. e...
Questions in other subjects:

Social Studies, 30.01.2020 15:49


Mathematics, 30.01.2020 15:49

English, 30.01.2020 15:49

Mathematics, 30.01.2020 15:49




Mathematics, 30.01.2020 15:49

History, 30.01.2020 15:49


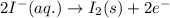
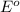 potential will always get reduced and will undergo reduction reaction.
potential will always get reduced and will undergo reduction reaction.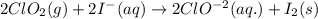
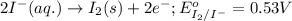
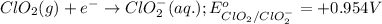 ( × 2 )
( × 2 )



