
Chemistry, 02.01.2020 22:31, Silkyruthie
Identify and label the brønsted-lowry acid, its conjugate base, the brønsted-lowry base, and its conjugate acid in each of the following equations:
(a) hno3+h2o⟶h3o++no3−
(b) cn− +h2o ⟶ hcn+oh−
(c) h2so4+cl− ⟶hcl+hso4−
(d) hso4−+oh− ⟶so42−+h2o
(e) o2− + h2 o ⟶ 2oh−

Answers: 2
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 21:30, starl0rd211
Describe at least two advantages and two disadvantages of using hydropower as a source of energy.
Answers: 2

Chemistry, 22.06.2019 23:00, emilyphillips1681
If two identical atoms are bonded, what kind of molecule is formed
Answers: 1
Do you know the correct answer?
Identify and label the brønsted-lowry acid, its conjugate base, the brønsted-lowry base, and its con...
Questions in other subjects:


Mathematics, 05.03.2021 09:40

English, 05.03.2021 09:40

History, 05.03.2021 09:40

Mathematics, 05.03.2021 09:40


Chemistry, 05.03.2021 09:40




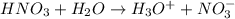
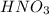 is Bronsted lowry acid and
is Bronsted lowry acid and 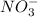 is its conjugate base.
is its conjugate base.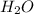 is Bronsted lowry base and
is Bronsted lowry base and 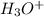 is its conjugate acid.
is its conjugate acid.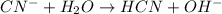
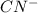 is Bronsted lowry base and HCN is its conjugate acid.
is Bronsted lowry base and HCN is its conjugate acid.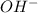 is its conjugate base.
is its conjugate base.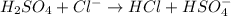
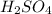 is Bronsted lowry acid and
is Bronsted lowry acid and 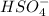 is its conjugate base.
is its conjugate base.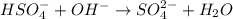
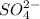 is its conjugate base.
is its conjugate base.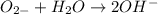
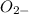 is Bronsted lowry base and OH- is its conjugate acid.
is Bronsted lowry base and OH- is its conjugate acid.



