
Calculate the standard entropy change for the industrial synthesis of urea (a common fertilizer): co2(g) + 2 nh3(g) → co(nh2)2(s) + h2o(ℓ) at 25◦c. patel (smp4358) – 28: fall exam review part three – lyssy – (112476) 8 s ◦ co2(g) 213.74 j k·mol nh3(g) 192.45 j k·mol co(nh2)2(s) 104.6 j k·mol h2o(ℓ) 69.91 j k·mol answer in units of j k · mol.

Answers: 2
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 04:00, fantasticratz2
Acontainer holds 35.8 moles of gas under 10.0 atm of pressure at 70.0 c. what is the volume of the container?
Answers: 2

Chemistry, 22.06.2019 07:40, caleb19moody
21. consider the following chemical reaction: n2+ o2 2 no if 10.0 g of n2 reacts with excess oxygen then how many grams of no can be formed? a) 10.7 g b) 21.4 g c) 32.9 g d) 42.8 g page 4 of 8
Answers: 2

Chemistry, 22.06.2019 13:20, alejandra340
Can someone me with 3 and 4 plz. this is for masteries test.
Answers: 2
Do you know the correct answer?
Calculate the standard entropy change for the industrial synthesis of urea (a common fertilizer): c...
Questions in other subjects:

Mathematics, 25.03.2021 18:20



Mathematics, 25.03.2021 18:20

Mathematics, 25.03.2021 18:20

English, 25.03.2021 18:20



Mathematics, 25.03.2021 18:20



 is:
is: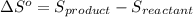
![\Delta S^o=[n_{CO(NH_2)_2}\times \Delta S^0_{(CO(NH_2)_2)}+n_{H_2O}\times \Delta S^0_{(H_2O)}]-[n_{CO_2}\times \Delta S^0_{(CO_2)}+n_{NH_3}\times \Delta S^0_{(NH_3)}]](/tpl/images/0438/1232/f3a9c.png)
 = entropy change of reaction = ?
= entropy change of reaction = ? = standard entropy change
= standard entropy change = 104.6 J/mol.K
= 104.6 J/mol.K = 69.91 J/mol.K
= 69.91 J/mol.K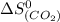 = 213.74 J/mol.K
= 213.74 J/mol.K = 192.45 J/mol.K
= 192.45 J/mol.K![\Delta S^o=[1mole\times (104.6J/K.mole)+1mole\times (69.91J/K.mole)}]-[1mole\times (213.74J/K.mole)+2mole\times (192.45J/K.mole)]](/tpl/images/0438/1232/6f6bf.png)





