

Answers: 2
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 12:00, macylen3900
Ineed this asap part i: scientific method what is the difference between science and pseudoscience? what is the scientific method?
Answers: 2

Do you know the correct answer?
Calculate the lattice energy of kcl(s) given the following data using the born-haber cycle: δhsubli...
Questions in other subjects:


History, 17.10.2021 14:00

Mathematics, 17.10.2021 14:00


Social Studies, 17.10.2021 14:00



Mathematics, 17.10.2021 14:00



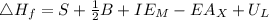
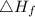 is enthalpy of formation
is enthalpy of formation 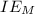 is ionisation enthalpy of metal
is ionisation enthalpy of metal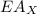 is electron affinity of non metal atom
is electron affinity of non metal atom 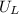 is lattice energy
is lattice energy 



