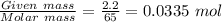Chemistry, 31.12.2019 05:31, carlosleblanc26
Zn + 2hcl → zncl2 + h2
at conditions of standard temperature and pressure, determine how many liters of hydrogen gas are produced by placing a zinc nail with a mass of 2.2g into an excess of hydrochloric acid.
a) 0.0015 l b) 0.75 l c) 1.33 l d) 6.42 l

Answers: 2
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 01:30, elizediax8683
(apex) when a cup of water is dropped, as the cup falls, the water in the cup falls out true or false?
Answers: 1

Chemistry, 22.06.2019 07:30, eburnhisel2023
The volume of helium in a blimp is 6.28 x 10^9 millimeters. the density of helium in the blimp is .1786 kilogram/meter^3. find the mass of the helium in the blimp.
Answers: 1
Do you know the correct answer?
Zn + 2hcl → zncl2 + h2
at conditions of standard temperature and pressure, determine how many...
at conditions of standard temperature and pressure, determine how many...
Questions in other subjects:




Mathematics, 24.06.2019 23:00


Mathematics, 24.06.2019 23:00

Mathematics, 24.06.2019 23:00

Mathematics, 24.06.2019 23:00

Mathematics, 24.06.2019 23:00