
The overall reaction 2co3+(aq) + 2cl-(aq) ? 2co2+(aq) + cl2(g) has the standard cell voltage eocell= 0.46 v.
given that cl2(g) + 2e-? 2cl-(aq), eo = 1.36 v,
calculate the standard reduction potential for the following the half reaction at 25oc:
co3+ + e-? co2+
1.82 v
-0.90 v
0.90 v
-1.82 v

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 19:00, eweqwoewoji
Agood hypothesis includes which of the following? a: prediction b: data c: uncertainty d: conclusion
Answers: 1


Chemistry, 21.06.2019 22:50, ajaydonlee
Select the correct answer how does the heat content of the reaction change in the process of photosynthesis when a glucose molecule is formed? ca the value of is negative the value of qis positive the value of a remains constant the value of a decreases the value of equals zero e
Answers: 2
Do you know the correct answer?
The overall reaction 2co3+(aq) + 2cl-(aq) ? 2co2+(aq) + cl2(g) has the standard cell voltage eocell...
Questions in other subjects:

Mathematics, 19.02.2021 01:10

Mathematics, 19.02.2021 01:10

World Languages, 19.02.2021 01:10


English, 19.02.2021 01:10

Mathematics, 19.02.2021 01:10



History, 19.02.2021 01:10


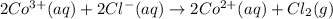
 ,
, 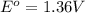
 ,
, 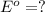
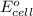 of this reaction is as follows.
of this reaction is as follows.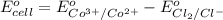
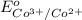 - 1.36 V
- 1.36 V



