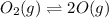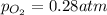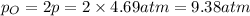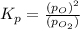Chemistry, 31.12.2019 04:31, angelespinosa521
Asample of o2(g) is placed in an otherwise empty, rigid container at 4224 k at an initial pressure of 4.97 atm, where it decomposes to o(g) by the reaction below.
o2(g) ⇄ 2 o(g)
at equilibrium, the partial pressure of o2 is 0.28 atm. calculate kp for this reaction at 4224 k.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:50, mrylenastewart
What are transitions between a liquid and a solid called? identify which way they are transitioning
Answers: 2

Chemistry, 22.06.2019 06:00, rigobertogarza2
According to each substances heat of fusion, which of the items below requires more heat to be added per gram of substance to go from solid to liquid? silver sulfur water lead
Answers: 2

Chemistry, 23.06.2019 02:00, Robloxdemonduckyt
As light moves from one material into the next, which of the following affects how much the light waves will refract, or bend? angle at which the ray strikes the medium color of the material density of the material temperature of the light wave
Answers: 2

Chemistry, 23.06.2019 02:00, sakria2002
What is the difference between a substance "getting wet" and "being dissolved" in a liquid at the particulate level?
Answers: 3
Do you know the correct answer?
Asample of o2(g) is placed in an otherwise empty, rigid container at 4224 k at an initial pressure o...
Questions in other subjects:

Social Studies, 07.07.2019 07:00

Computers and Technology, 07.07.2019 07:00

Mathematics, 07.07.2019 07:00

Mathematics, 07.07.2019 07:00

English, 07.07.2019 07:00

English, 07.07.2019 07:00

Mathematics, 07.07.2019 07:00




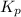 at 4224 K is 314.23.
at 4224 K is 314.23.