
Chemistry, 30.12.2019 23:31, 6224968918
Outline the steps needed to determine the limiting reactant when 30.0 g of propane, c3h8, is burned with 75.0 g of oxygen.
percent yield = 0.8347g / 0.9525 g × 100% = 87.6%

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 18:00, ambarpena14
An object displaces 652 ml of water. the volume of the object is: 0.652 cm³ 6.52 cm³ 65.2 cm³ 652 cm³
Answers: 2


Chemistry, 23.06.2019 00:00, familyk0jj3
How do you determine the percent yield of a chemical reaction
Answers: 1

Chemistry, 23.06.2019 00:30, terryg4397
Fred is studying a substance that is made out of only one element. this means that
Answers: 1
Do you know the correct answer?
Outline the steps needed to determine the limiting reactant when 30.0 g of propane, c3h8, is burned...
Questions in other subjects:



English, 16.10.2019 22:00


Physics, 16.10.2019 22:00




Mathematics, 16.10.2019 22:00


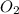
 = 30.0 g
= 30.0 g

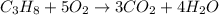
 moles of
moles of 



