
At equilibrium, the concentrations of the products and reactants for the reaction, h2 (g) + i2 (g) 2 hi (g), are [h2] = 0.106 m; [i2] = 0.022 m; [hi] = 1.29 m calculate the new equilibrium concentration of hi (in m) if the equilibrium concentrations of h2 and i2 are 0.95 m and 0.019 m respectively.

Answers: 3
Other questions on the subject: Chemistry


Chemistry, 21.06.2019 23:00, dice50
When determining the shape of a molecule, it is important to draw a lewis dot structure first in order to see the total number a. electrons within the moleculeb. bonding and unshared pairs around central atomc. unshared pair within the molecule( i really need it )
Answers: 1

Chemistry, 22.06.2019 06:00, lindseyklewis1p56uvi
Ethanol (c2h5oh) is produced from the fermentation of sucrose in the presence of enzymes. c12h22o11(aq) + h2o(g) 4 c2h5oh(l) + 4 co2(g) determine the theoretical yield and the percent yields of ethanol if 680. g sucrose undergoes fermentation and 326.5 g ethanol is obtained. theoretical _ g _ percent %
Answers: 1
Do you know the correct answer?
At equilibrium, the concentrations of the products and reactants for the reaction, h2 (g) + i2 (g) ...
Questions in other subjects:





Mathematics, 15.12.2020 21:30




Geography, 15.12.2020 21:30


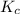 ) for the given chemical reaction, is given by the equation:
) for the given chemical reaction, is given by the equation:![K_{c} = \frac {[HI]^{2}}{[H_{2}]\: [I_{2}]}](/tpl/images/0435/6174/f8601.png)
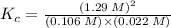
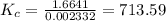
![K_{c} = \frac {[HI]^{2}}{(0.95\: M) \times (0.019\: M)}](/tpl/images/0435/6174/d2511.png)
![\Rightarrow K_{c} = 713.59 = \frac {[HI]^{2}}{0.01805}](/tpl/images/0435/6174/fef3c.png)
![\Rightarrow [HI]^{2} = 713.59 \times 0.01805 = 12.88](/tpl/images/0435/6174/d4ab1.png)
![\Rightarrow [HI] = \sqrt {12.88} = 3.589 M](/tpl/images/0435/6174/5120a.png)





