
Chemistry, 28.12.2019 05:31, nkazmirski5598
Consider a voltaic cell where the anode half-reaction is zn(s) → zn2+(aq) + 2 e− and the cathode half-reaction is sn2+(aq) + 2 e– → sn(s). what is the concentration of sn2+ if zn2+ is 2.5 × 10−3 m and the cell emf is 0.660 v? the standard reduction potentials are given below
zn+2(aq) + 2 e−→ zn(s) e∘red = −0.76 v
sn2+(aq) + 2 e– →sn(s) e∘red =-0.136 v
a. 9.0*10^-3 m
b. 3..3*10^-2 m
c. 6.9*10^-4 m
d. 7.6*10^-3 m

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 01:30, MickeyxX7096
Phosphorous acid, h3po3(aq) , is a diprotic oxyacid that is an important compound in industry and agriculture. the values of phosphorous acid are 1.30 6.70 calculate the ph for each of the given points in the titration of 50.0 ml of 1.5 m h3po3(aq) with 1.5 m koh(aq) .
Answers: 3


Chemistry, 22.06.2019 08:30, Blaise2653
Joan writes four numbers on the board in standard form, and then she writes their scientific notation
Answers: 1

Chemistry, 22.06.2019 08:30, myamiller558
Which of the following would be an accurate picture of the earth during the summer time of the northern hemisphere?
Answers: 1
Do you know the correct answer?
Consider a voltaic cell where the anode half-reaction is zn(s) → zn2+(aq) + 2 e− and the cathode hal...
Questions in other subjects:


Biology, 17.03.2021 23:40


Computers and Technology, 17.03.2021 23:40

Mathematics, 17.03.2021 23:40

Mathematics, 17.03.2021 23:40

World Languages, 17.03.2021 23:40

Mathematics, 17.03.2021 23:40

Spanish, 17.03.2021 23:40


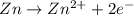

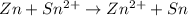
 :
:![E_{cell}=(E_{Sn^{2+}\mid Sn}^{0}-E_{Zn^{2+}\mid Zn}^{0})-\frac{0.059}{n}log\frac{[Zn^{2+}]}{[Sn^{2+}]}](/tpl/images/0435/4933/a904f.png)
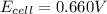 and
and ![[Zn^{2+}]=2.5\times 10^{-3}M](/tpl/images/0435/4933/eef66.png)
![0.660V=(-0.136V+0.76V)-\frac{0.059}{2}log\frac{2.5\times 10^{-3}M}{[Sn^{2+}]}](/tpl/images/0435/4933/821cc.png)
![[Sn^{2+}]=4.2\times 10^{-2}M](/tpl/images/0435/4933/6b94f.png)
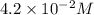 is most closest to
is most closest to 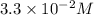 therefore option (B) is correct.
therefore option (B) is correct.




