
Chemistry, 28.12.2019 05:31, hannah1072
Consider a mystery compound having the formula mxty. if the compound is not an acid, if it contains only two elements, and if m is not a metal, which of the following is true about the compound? a.) it contains a polyatomic ion. b.) its name ends in -ite or -ate. c.) its name ends in -ic. d.) it is a binary molecular compound.

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 10:00, melissa9882
A50.0g sample of liquid water at 0.0 c ends up as ice at -20.0 c. how much energy is involved in this change?
Answers: 1


Chemistry, 22.06.2019 22:30, darceline1574
Vi limitens. vastery test select the correct answer. which statement explains why large atoms are more reactive than small atoms? a. large atoms have valence electrons farther from the nucleus and lose them more readily. b. large atoms have greater ionization energy, which they can utilize during a reaction. c. large atoms have a greater number of electrons that they can lose during a reaction. d. large atoms have more energy levels, so they have more energy to pass on in a reaction. reset next
Answers: 3
Do you know the correct answer?
Consider a mystery compound having the formula mxty. if the compound is not an acid, if it contains...
Questions in other subjects:

Mathematics, 12.12.2020 16:00


Chemistry, 12.12.2020 16:00



Advanced Placement (AP), 12.12.2020 16:00

History, 12.12.2020 16:00


Mathematics, 12.12.2020 16:00


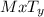 . The compound is not acidic in nature and the element 'M' is not a metal. This shows that the compound does not contain any metal. Based on the definition of a binary molecular compound as a compound comprising elements that are not metals. Therefore, the compound is obviously a binary molecular compound.
. The compound is not acidic in nature and the element 'M' is not a metal. This shows that the compound does not contain any metal. Based on the definition of a binary molecular compound as a compound comprising elements that are not metals. Therefore, the compound is obviously a binary molecular compound.



