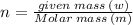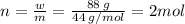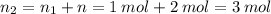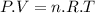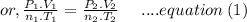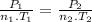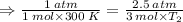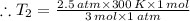Chemistry, 28.12.2019 04:31, hilljade45
Aflask of fixed volume contains 1.0 mole of gaseous carbon dioxide and 88 g of solid carbon dioxide. the original pressure and temperature in the flask is 1.0 atm and 300. k. all of the solid carbon dioxide sublimes. the final pressure in the flask is 2.5 atm. what is the final temperature? assume the solid carbon dioxide takes up negligible volume.
a. 150 k
b. 200 k
c. 250 k
d. 300 k
e. 400 k

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 17:30, mwest200316
To find the enthalpy of a reaction in the lab, you measured the of the reactants and the change during the reaction.
Answers: 1

Chemistry, 23.06.2019 01:30, Sonicawesomeness
Select the correct answer from each drop-down menu. to make a table of the elements, dmitri mendeleev sorted the elements according to their . he then split the list of elements into several columns so that elements beside each other had similar .
Answers: 2
Do you know the correct answer?
Aflask of fixed volume contains 1.0 mole of gaseous carbon dioxide and 88 g of solid carbon dioxide....
Questions in other subjects:

English, 06.06.2020 22:57


History, 06.06.2020 22:57

History, 06.06.2020 22:57

Mathematics, 06.06.2020 22:57




English, 06.06.2020 22:57

Mathematics, 06.06.2020 22:57