
Chemistry, 28.12.2019 02:31, appattuvilai1234
Solid copper (ii) oxide is placed in a sealed container with an excess of ammonia gas. when equilibrium is established the partial pressure of n2 is 0.255atm. the reaction is as follows: 3cuo (s) + 2 nh3 (g) ↔ 3 cu (s) + n2 (g) + 3 h2o (g) a) what is the partial pressure of h2o (g) at equilibrium?

Answers: 2
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 00:30, mannster03
This active feature of earth's crust in building mountain ranges as well as islands. this feature is a a) cavern. b) earthquake. c) mountain. d) volcano.
Answers: 2

Chemistry, 22.06.2019 07:00, daniellekennedy05
If there is any 12 to 14 girls that need a boyfriend just follow me and let me know
Answers: 1

Chemistry, 22.06.2019 10:00, zionlopez543
Americium-241 undergoes fission to produce three neutrons per fission event. if a neutron-absorbing material is mixed in with this sample so that the rate of neutron production drops down to 1.8 neutrons per fission event, which will be effective at achieving a critical mass? check all that apply. remove a deflective shield surrounding the sample. remove absorbent material mixed in with the sample. compress the sample of americium-241.
Answers: 1
Do you know the correct answer?
Solid copper (ii) oxide is placed in a sealed container with an excess of ammonia gas. when equilibr...
Questions in other subjects:

Mathematics, 02.03.2021 23:40

Mathematics, 02.03.2021 23:40




Mathematics, 02.03.2021 23:40

English, 02.03.2021 23:40

English, 02.03.2021 23:40

English, 02.03.2021 23:40

Business, 02.03.2021 23:40


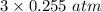 = 0.765 atm
= 0.765 atm



