

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 14:00, hannahhoskings6989
What was bohr’s contribution to the planetary model
Answers: 1

Do you know the correct answer?
Consider the following standard heats of formation:
p₄o₁₀(s) = -3110 kj/mol
h₂o(l) =...
p₄o₁₀(s) = -3110 kj/mol
h₂o(l) =...
Questions in other subjects:

Mathematics, 18.09.2020 22:01

Mathematics, 18.09.2020 22:01

Mathematics, 18.09.2020 22:01

Mathematics, 18.09.2020 22:01

History, 18.09.2020 22:01

Mathematics, 18.09.2020 22:01

Mathematics, 18.09.2020 22:01

Mathematics, 18.09.2020 22:01

Mathematics, 18.09.2020 22:01

Mathematics, 18.09.2020 22:01


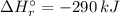
![\Delta H_{f}^{\circ } [P_{4}O_{10}(s)]](/tpl/images/0435/2502/5b047.png) = -3110 kJ/mol,
= -3110 kJ/mol,![\Delta H_{f}^{\circ } [H_{2}O(l)]](/tpl/images/0435/2502/46f61.png) = -286 kJ/mol,
= -286 kJ/mol, ![\Delta H_{f}^{\circ } [H_{3}PO_{4}(s)]](/tpl/images/0435/2502/1d826.png) = -1279 kJ/mol
= -1279 kJ/mol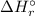 = ?
= ?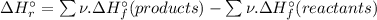
![\Delta H_{r}^{\circ } = [4 \times \Delta H_{f}^{\circ } [H_{3}PO_{4}(s)]] - [1 \times \Delta H_{f}^{\circ } [P_{4}O_{10}(s)] + 6 \times \Delta H_{f}^{\circ } [H_{2}O(l)]]](/tpl/images/0435/2502/c9ebf.png)
![\Rightarrow \Delta H_{r}^{\circ } = [4 \times (-1279\, kJ/mol)] - [1 \times (-3110\, kJ/mol) + 6 \times (-286\, kJ/mol)]](/tpl/images/0435/2502/ea7a2.png)
![\Rightarrow \Delta H_{r}^{\circ } = [-5116\, kJ] - [-3110\, kJ -1716\, kJ]](/tpl/images/0435/2502/7e6ce.png)
![\Rightarrow \Delta H_{r}^{\circ } = [-5116\, kJ] - [-4826\, kJ] = -290\,kJ](/tpl/images/0435/2502/ee612.png)




