
Chemistry, 28.12.2019 00:31, quincyjosiah07
How much heat is absorbed/released when 20.00 g of nh3(g) reacts in the presence of excess o2 (g) to produce no (g) and h2o (l) according to the following chemical equation? 4nh3 (g) + 5o2 (g) > 4no (g) +6h2o (l) δ h: +1168 kja. 342.9 kj of heat are absorbed. b. 342.9 kj of heat are released. c. 1372 kj of heat are absorbed. d. 1372 kj of heat are released.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 21:40, MathChic68
It is important to wear proper protective equipment in lab even when not actively performing experiments because accidents can affect any researcher, even one not working on an experiment. select the best answer from the choices provided
Answers: 3

Chemistry, 22.06.2019 04:00, nothingworksoutforme
The image shows a process that releases nuclear energy which statement best identifies the process shown the process must be fusion because energy is released the process must be fusion because of have your nucleus formed a smaller nuclei the process must be fission because a large nucleus breaks into smaller nuclei the process must be fission because neutrons are formed
Answers: 1

Chemistry, 22.06.2019 05:50, zaleemawhite
Significant figures are digits read directly from the measuring instrument plus one more digit, which is __ by the observer.
Answers: 2

Chemistry, 22.06.2019 11:30, ansuaprajita1506
Voltaic cells produce a positive overall charge. what does this indicate? a. the reaction is likely to be endothermic. b. the reaction is spontaneous. c. the reaction is not likely to occur. d. the reaction is not spontaneous.
Answers: 3
Do you know the correct answer?
How much heat is absorbed/released when 20.00 g of nh3(g) reacts in the presence of excess o2 (g) to...
Questions in other subjects:


Health, 31.10.2020 03:40




History, 31.10.2020 03:40

History, 31.10.2020 03:40


Mathematics, 31.10.2020 03:40

Mathematics, 31.10.2020 03:40


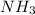 as:-
as:-
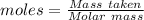
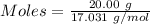
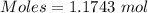
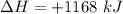
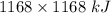 of heat
of heat



