

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 21:00, bakoeboo
Harvey kept a balloon with a volume of 348 milliliters at 25.0˚c inside a freezer for a night. when he took it out, its new volume was 322 milliliters, but its pressure was the same. if the final temperature of the balloon is the same as the freezer’s, what is the temperature of the freezer? the temperature of the freezer is kelvins.
Answers: 2

Chemistry, 22.06.2019 10:50, lejeanjamespete1
8) a mixture of he, ne and ar has a pressure of 7.85 atm. if the ne has a mole fraction of 0.47 and 8) ar has a mole fraction of 0.23, what is the pressure of he? a) 4.2 atm b) 3.7 atm c) 5.5 atm d) 2.4 atm e) 1.8 atm
Answers: 1

Do you know the correct answer?
For the reaction, 2cr2+ + cl2(g) > 2cr3+ + 2cl- e cell (standard conditions) = 1.78v calculate e...
Questions in other subjects:


Chemistry, 18.03.2021 01:40

Biology, 18.03.2021 01:40

History, 18.03.2021 01:40

Business, 18.03.2021 01:40



History, 18.03.2021 01:40


Mathematics, 18.03.2021 01:40


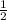 . It doesn't influence the value of the cell potential.
. It doesn't influence the value of the cell potential.



