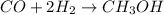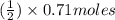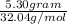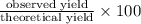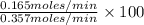Chemistry, 27.12.2019 05:31, lorelei7668
Methanol (ch3oh) can be produced by the following reaction: co(g) 1 2h2(g) 88n ch3oh(g) hydrogen at stp flows into a reactor at a rate of 16.0 l/min. carbon monoxide at stp flows into the reactor at a rate of 25.0 l/min. if 5.30 g of methanol is produced per minute, what is the percent yield of the reaction?

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:00, NREYESLDS2806
To save time, you can approximate the initial mass of the solid to the nearest ±1 g. for example, if you are asked to add 14.3 g of copper, add between 13 g and 15 g. which of the following sets include two samples with an equal density? which all that apply below 15.4 g gold and 18.7 g silver 15.2 g copper and 50.0 g copper 20.2 g silver and 20.2 g copper 11.2 g gold and 14.9 g gold
Answers: 1


Chemistry, 22.06.2019 10:00, alexabdercmur
Why is the structure of molecule important to its function?
Answers: 1

Chemistry, 22.06.2019 11:50, bellojamilet410
What substance has a mass of 9.5g and volume of 2.1cm^3
Answers: 2
Do you know the correct answer?
Methanol (ch3oh) can be produced by the following reaction: co(g) 1 2h2(g) 88n ch3oh(g) hydrogen at...
Questions in other subjects:











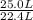 per minute
per minute
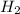 being fed per minute =
being fed per minute = 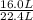 per minute
per minute