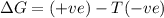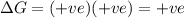Acertain process has δh° > 0, δs° < 0, and δg° > 0. the values of δh° and δs° do not depend on the temperature. which of the following is a correct conclusion about this process? none of the above conclusions is correct. it is non-spontaneous at all t. it is spontaneous at low t. it is spontaneous at all t. it is spontaneous at high t

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 18:00, Jazmineboo7709
Alidded glass container is filled with a colored gas. after a period of time, it is observed that the gas is uniformly spread throughout the box and that the movement has slowed considerably. next, a warm iron plate is carefully placed under the box. why is there resumed movement of the gas in the container?
Answers: 2

Chemistry, 22.06.2019 20:30, demarcuswiseman
From the choices provided below, list the reagent(s) in order that will react with cyclopentanone to form the compound shown below.
Answers: 2

Chemistry, 23.06.2019 05:00, mprjug6
In 1901, thomas edison invented the nickel-iron battery. the following reaction takes place in the battery. fe(s) + 2 nio(oh)(s) + 2 h2o(l) fe(oh)2(s) + 2 ni(oh)2(aq) how many mole of fe(oh)2, is produced when 4.20 mol fe and 6.70 mol nio(oh) react?
Answers: 3
Do you know the correct answer?
Acertain process has δh° > 0, δs° < 0, and δg° > 0. the values of δh° and δs° do not dep...
Questions in other subjects:


Biology, 27.07.2019 18:30


Social Studies, 27.07.2019 18:30



Biology, 27.07.2019 18:30

Mathematics, 27.07.2019 18:30


Social Studies, 27.07.2019 18:30


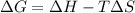
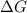 = Gibbs free energy = +ve
= Gibbs free energy = +ve
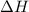 = enthalpy change = +ve
= enthalpy change = +ve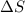 = entropy change = -ve
= entropy change = -ve