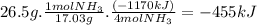Chemistry, 27.12.2019 04:31, mstahuggoo6102
Ammonia undergoes combustion to yield nitric oxide and water by the following reaction equation: 4 nh3 (g) + 5 o2 (g) → 4 no (g) + 6 h2o (g) δh = - 1170 kjif 26.5 g of nh3 is reacted with excess o2, what will be the amount of heat given off?

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 13:00, Savadt2810
What is the ph of a solution with a concentration of 5.2 × 10–8 m h3o+?
Answers: 1

Chemistry, 22.06.2019 12:30, ethanw8973
If 22.5 liters of oxygen reacted with excess of hydrogen, how many liters of water vapor could be produced?
Answers: 3
Do you know the correct answer?
Ammonia undergoes combustion to yield nitric oxide and water by the following reaction equation: 4...
Questions in other subjects:


History, 20.10.2019 02:00

Mathematics, 20.10.2019 02:00

Biology, 20.10.2019 02:00

Mathematics, 20.10.2019 02:00

Spanish, 20.10.2019 02:00