
Chemistry, 27.12.2019 04:31, triciajfive
Suppose hydrochloric acid reacts with potassium sulfite yielding water, sulfur dioxide, and potassium chloride. suppose 4 moles of hydrochloric acid react with excess potassium sulfite. how many grams of sulfur dioxide are produced?

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:00, natalie857123
For each of the following types of reactions, write a general reaction formula in the symbolic form—for example, a + b → ab. single-displacement double-displacement synthesis decomposition
Answers: 1


Chemistry, 22.06.2019 17:00, brandiwingard
What is the mass of phosphorous in a 51-kg person
Answers: 1

Chemistry, 22.06.2019 18:00, kingamir
Answer asap need it by wednesday morning carry out the following calculations on ph and ka of from data. i. calculate the ph of 0.02m hcl ii. calculate the ph of 0.036m naoh iii. calculate the ph of 0.36m ca(oh)2 iv. calculate the ph of 0.16m ch3cooh which has ka = 1.74 x 10-5 mol dm-3 v. calculate ka for weak acid ha which has a ph of 3.65 at 0.30m concentration vi. calculate the ka of a solution made by mixing 15.0 cm3 0.2m ha and 60.0 cm3 0.31m a-. [ph= 3.80] vii. calculate the ph of a solution made by mixing 15.0 cm3 0.1m naoh and 35.0 cm3 0.2m hcooh. [ka = 1.82 x 10-4 m]
Answers: 1
Do you know the correct answer?
Suppose hydrochloric acid reacts with potassium sulfite yielding water, sulfur dioxide, and potassiu...
Questions in other subjects:




Mathematics, 27.06.2019 15:00

Mathematics, 27.06.2019 15:00



Social Studies, 27.06.2019 15:00


Mathematics, 27.06.2019 15:00


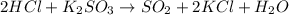
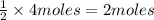 of sulfur dioxide gas.
of sulfur dioxide gas.



