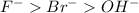Chemistry, 27.12.2019 02:31, RosaJackson8088
Consider four different samples: aqueous nabr, molten nabr, aqueous naf, and molten naf. current run through each sample produces one of the following products at the anode: liquid bromine, fluorine gas, or oxygen gas. match each sample to its anodic product.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 17:10, PSBSolarYT
For which one of the following reactions is the value of δh° rxn equal to δh° f for the product? a. 2 h2 (g) + o2 (g) → 2 h2o (l) b. n2 (g) + o2 (g) → 2 no (g) c. 2 h2 (g) + o2 (g) → 2 h2o (g) d. h2o (l) + 1/2 o2 (g) → h2o2 (l) e. none of the above
Answers: 1

Chemistry, 22.06.2019 09:00, kkmonsterhigh18
The diagram below shows a cell placed in a solution. a cell is shown placed inside a beaker. it is labeled cell. the solution inside the beaker is labeled 40% salt solution and the solution inside the cell is labeled 20% salt solution. only water is allowed to move in and out of the cell. what will most likely happen to the cell? it will expand as water moves out of it. it will shrink as water moves out of it. it will expand as water moves into it. it will shrink as water moves into it.
Answers: 2

Chemistry, 22.06.2019 15:50, Edwardwall
Elements in group 2 are all called alkaline earth metals. what is most similar about the alkaline earth metals?
Answers: 1

Chemistry, 23.06.2019 01:00, williedenmark42
What is the most common form of matter in the universe
Answers: 2
Do you know the correct answer?
Consider four different samples: aqueous nabr, molten nabr, aqueous naf, and molten naf. current ru...
Questions in other subjects:




Mathematics, 10.11.2020 23:40


Chemistry, 10.11.2020 23:40