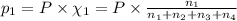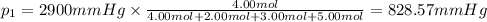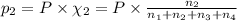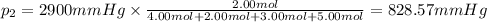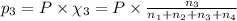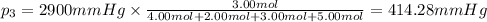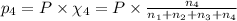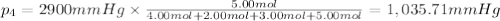Chemistry, 27.12.2019 02:31, ahmedeldyame
Amixture of gases consists of 4.00 moles of he, 2.00 moles of h2, 3.00 moles of co2 and 5.00 moles of ar. the total pressure of the mixture is 2900 mm. determine the mole fraction of each gas in the mixture. determine the mole percent of each gas in the mixture. determine the partial pressure of each gas in the mixture.

Answers: 2
Other questions on the subject: Chemistry



Chemistry, 22.06.2019 08:00, juliannxkim
Joe shines white light into a bowl half full of water at an angle of incident of 27.5°. calculate the angle of refraction in the water given the indices of refraction for air and water are 1.00 and 1.36, respectively.
Answers: 2

Chemistry, 22.06.2019 20:20, carcon2019
Nitric acid can be formed in two steps from the atmospheric gases nitrogen and oxygen, plus hydrogen prepared by reforming natural gas. in the first step, nitrogen and hydrogen react to form ammonia: (g) (g) (g) in the second step, ammonia and oxygen react to form nitric acid and water: (g) (g) (g) (g) calculate the net change in enthalpy for the formation of one mole of nitric acid from nitrogen, hydrogen and oxygen from these reactions. round your answer to the nearest .
Answers: 3
Do you know the correct answer?
Amixture of gases consists of 4.00 moles of he, 2.00 moles of h2, 3.00 moles of co2 and 5.00 moles o...
Questions in other subjects:

Mathematics, 25.03.2021 07:50



Mathematics, 25.03.2021 07:50

Mathematics, 25.03.2021 07:50

Social Studies, 25.03.2021 07:50



Mathematics, 25.03.2021 07:50


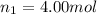
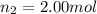
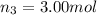
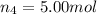
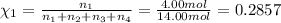
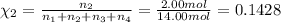
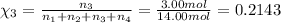
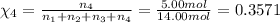
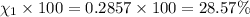
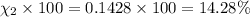
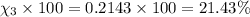

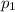
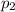
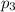
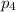
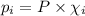
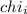 = Mole fraction of ith component
= Mole fraction of ith component