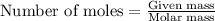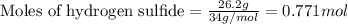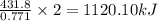Chemistry, 27.12.2019 00:31, lestessanders02
Hydrosulfuric acid (h2s) undergoes combustion to yield sulfur dioxide and water by the following reaction equation: 2 h2s + 3 o2 → 2 so2 + 2 h2o

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 12:30, UaRemomGAY
If anyone would be able to me out with these three questions it would be these are from the chem 2202 course.
Answers: 3

Chemistry, 22.06.2019 16:50, TheOriginal2x
Assuming complete dissociation of the solute, how many grams of kno3 must be added to 275 ml of water to produce a solution that freezes at -14.5 c? the freezing point for pure water is 0.0 c and k_f is equal to 1.86 c/m
Answers: 3

Chemistry, 22.06.2019 18:00, jeepjose58
What amount of heat is exchanged when 106.2 grams of substance y goes from a liquid at 35 degrees celsius to a solid at the same temperature? melting point of substance y = 35 degrees c; δhvaporization = 3.67 j/mol; δhfusion = 3.30 j/mol. mwsubstance y = 28.22 g/mol. −12.4 j −3.51 x 102 j 1.24 x 101 j 351 j
Answers: 1

Chemistry, 22.06.2019 21:30, djdjdjdbdbjx
What is another way to determine mass times acceleration?
Answers: 1
Do you know the correct answer?
Hydrosulfuric acid (h2s) undergoes combustion to yield sulfur dioxide and water by the following rea...
Questions in other subjects:



English, 25.09.2019 21:00

Mathematics, 25.09.2019 21:00





English, 25.09.2019 21:00


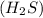 undergoes combustion to yield sulfur dioxide and water by the following reaction equation:
undergoes combustion to yield sulfur dioxide and water by the following reaction equation: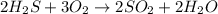
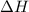 of the reaction if 26.2 g of
of the reaction if 26.2 g of 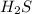 reacts with excess
reacts with excess 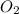 to yield 431.8 kJ?
to yield 431.8 kJ?