
Chemistry, 27.12.2019 00:31, ehuntsman8221
Asolution is prepared by dissolving 0.56 g of benzoic acid (c6h5co2h, ka 6.4 ) in enough water to make 1.0 l of solution. calculate [c6h5co2h], , , , and the ph of this solution.

Answers: 2
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 06:30, dpchill5232
Suppose a lab group reports a ppercent yield of sand of 105. is it really possible to collect more sand than was originally represented? what is the possible explanation for the extra product?
Answers: 2


Chemistry, 22.06.2019 17:00, calmicaela12s
Which statement is true about a catalyst? a: a catalyst decreases the rate of the reaction. b. a catalyst is consumed during a chemical reaction. c. a catalyst lowers the activation energy of a reaction. d. a catalyst increases the reactant concentration during a reaction.
Answers: 1
Do you know the correct answer?
Asolution is prepared by dissolving 0.56 g of benzoic acid (c6h5co2h, ka 6.4 ) in enough water to...
Questions in other subjects:

Mathematics, 15.12.2020 23:20





Mathematics, 15.12.2020 23:20

Mathematics, 15.12.2020 23:20




![$\left[\mathrm{H}^{+}\right]=\left[\mathrm{C}_{6} \mathrm{H}_{5} \mathrm{CO}_{2}^{-}\right]=5.1 \times 10^{-4} \mathrm{M}$](/tpl/images/0434/1977/10f45.png)
![$\left[\mathrm{C}_{6} \mathrm{H}_{5} \mathrm{CO}_{2} \mathrm{H}\right]=4.1 \times 10^{-3} \mathrm{M}$](/tpl/images/0434/1977/9a3e2.png)
![$\left[\mathrm{OH}^{-}\right]=1.9 \times 10^{-11} \mathrm{M}](/tpl/images/0434/1977/e5b4e.png)

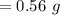

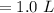
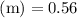

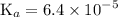
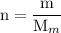
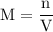
 and the number of moles (n) of benzoic acid:
and the number of moles (n) of benzoic acid:



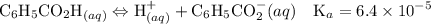
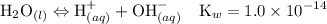
![$\mathrm{K}_{a}=\frac{\left[\mathrm{H}^{+}\right]\left[\mathrm{C}_{6} \mathrm{H}_{5} \mathrm{CO}_{2}^{-}\right]}{\left[\mathrm{C}_{6} \mathrm{H}_{5} \mathrm{CO}_{2} \mathrm{H}\right]}=6.4 \times 10^{-5}$](/tpl/images/0434/1977/e74c4.png)
![$\left[\mathrm{H}^{+}\right]_{0}$](/tpl/images/0434/1977/f38bd.png) is 0. (because of autonization)
is 0. (because of autonization)![$\left[\mathrm{H}^{+}\right]_{0}=10^{-7} \mathrm{M} \approx 0$](/tpl/images/0434/1977/a3a3d.png)
![$\left[\mathrm{C}_{6} \mathrm{H}_{5} \mathrm{CO}_{2} \mathrm{H}\right]=\left[\mathrm{C}_{6} \mathrm{H}_{5} \mathrm{CO}_{2} \mathrm{H}\right]_{0}-\mathrm{x}=0.00459-\mathrm{x}$](/tpl/images/0434/1977/a086a.png)
![$\left[\mathrm{C}_{6} \mathrm{H}_{5} \mathrm{CO}_{2}^{-}\right]=\left[\mathrm{C}_{6} \mathrm{H}_{5} \mathrm{CO}_{2}^{-}\right]_{0}+\mathrm{x}=0+\mathrm{x}=\mathrm{x}$](/tpl/images/0434/1977/89a6e.png)
![$\left[\mathrm{H}^{+}\right]=\left[\mathrm{H}^{+}\right]_{0}+x=0+x=x$](/tpl/images/0434/1977/2b64c.png)

![$\left[\mathrm{H}^{+}\right]$](/tpl/images/0434/1977/93e23.png) is much smaller than
is much smaller than  to decide whether the following approximation is valid or not:
to decide whether the following approximation is valid or not:


![$\mathrm{x}=4.9 \times 10^{-4}=\left[\mathrm{H}^{+}\right]$\\](/tpl/images/0434/1977/329c6.png)

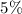 of acid is dissociated.
of acid is dissociated. :
: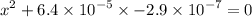
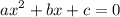

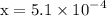
![\mathrm{M}=\left[\mathrm{H}^{+}\right]=\left[\mathrm{C}_{6} \mathrm{H}_{5} \mathrm{CO}_{2}^{-}\right]$](/tpl/images/0434/1977/6835a.png)
![$\left[\mathrm{C}_{6} \mathrm{H}_{5} \mathrm{CO}_{2} \mathrm{H}\right]=0.00459-0.00051=4.1 \times 10^{-3} \mathrm{M}$](/tpl/images/0434/1977/3ea5f.png)
![$\left[\mathrm{OH}^{-}\right]=\frac{\mathrm{K}_{w}}{\left[\mathrm{H}^{+}\right]}=\frac{1.0 \times 10^{-14}}{5.1 \times 10^{-4}}=1.9 \times 10^{-11} \mathrm{M}$](/tpl/images/0434/1977/7340a.png)
![$\mathrm{pH}=-\log \left[\mathrm{H}^{+}\right]=-\log \left(5.1 \times 10^{-4}\right)=3.29$](/tpl/images/0434/1977/3ad18.png)





