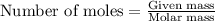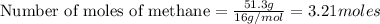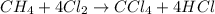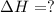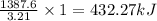Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 08:30, kkelley9223
How would the number of moles (n) of o2 change if the atmospheric pressure doubled but all other variables stayed the same
Answers: 2


Chemistry, 22.06.2019 09:20, payshencec21
What happened to the amount of carbon dioxide in the atmosphere from 2010–2017?
Answers: 1
Do you know the correct answer?
Methane (ch4) reacts with cl2 to yield ccl4 and hcl by the following reaction equation: ch4 + 4 cl2...
Questions in other subjects:



Mathematics, 05.11.2020 18:30








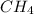 reacts with excess
reacts with excess 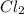 to yield 1387.6 kJ is 432.27kJ
to yield 1387.6 kJ is 432.27kJ