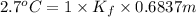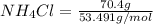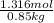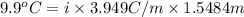When of benzamide are dissolved in of a certain mystery liquid , the freezing point of the solution is less than the freezing point of pure . calculate the mass of ammonium chloride that must be dissolved in the same mass of to produce the same depression in freezing point. the van't hoff factor for ammonium chloride in .

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:20, barry14201
Identify and describe the three ways that mutations affect organisms.
Answers: 1

Chemistry, 22.06.2019 06:00, giusto1894
24. a sports ball is inflated to an internal pressure of 1.85 atm at room temperature (25 °c). if the ball is then played with outside where the temperature is 7.5 °c, what will be the new pressure of the ball? assume the ball does not change in volume nor does any air leak from the ball a) 0.555 atm b) 1.74 atm c) 1.85 atm d) 1.97 atm
Answers: 2

Chemistry, 22.06.2019 07:00, coolkid2041
Calculate the number of moles of ethane in 100 grams
Answers: 3
Do you know the correct answer?
When of benzamide are dissolved in of a certain mystery liquid , the freezing point of the solution...
Questions in other subjects:





Mathematics, 05.11.2019 13:31





Mathematics, 05.11.2019 13:31


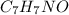 ) are dissolved in 850 g of a certain mystery liquid X, the freezing point of the solution is
) are dissolved in 850 g of a certain mystery liquid X, the freezing point of the solution is 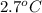 lower than the freezing point of pure X. On the other hand, when 70.4 g of ammonium chloride (
lower than the freezing point of pure X. On the other hand, when 70.4 g of ammonium chloride (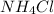 ) are dissolved in the same mass of X, the freezing point of the solution is
) are dissolved in the same mass of X, the freezing point of the solution is 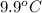 lower than the freezing point of pure X.
lower than the freezing point of pure X.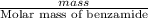
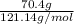
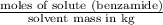
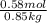
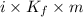 ,
, 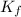 = freezing point constant of solvent
= freezing point constant of solvent