
Chemistry, 26.12.2019 21:31, brandonthomas11
A2.5 l flask is filled with 0.25 atm so3, 0.20 atm so2, and 0.40 atm o2, and allowed to reach equilibrium. assume at the temperature of the mixture is chosen so that kp = 0.12. predict the effect on the partial pressure of so3 as equilibrium is achieved by using q, the reaction quotient.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 18:00, thechocolatblanc
During which movies do spring tides new moon first quarter waxing gibbous waxing
Answers: 1

Chemistry, 21.06.2019 21:50, kyleighmarie05
H2so4(aq) + mg(s)—> mgso4(aq) +h2(g) which substance is the acid in the reaction?
Answers: 3

Chemistry, 22.06.2019 00:30, BLASIANNkidd
Maria wants to determine which type of disinfectant kills the most bacteria. which of the following is the best way for maria to determine this? a. ask ten different companies that make disinfectants which type is best. b. put the same amount and species of bacteria on ten identical plates, and add ten different kinds of disinfectant to each plate. c. interview ten different people to determine which type of disinfectant they prefer. d. put the same amount and species of bacteria on ten identical plates, and add a different disinfectant to each plate.
Answers: 1

Chemistry, 22.06.2019 23:10, RealStephani
Match the formula for the following compound: magnesium sulfate heptahydratemgs·7h2omg2so4·7h2omg(s o4)2·7h2omgso4·7h2o
Answers: 1
Do you know the correct answer?
A2.5 l flask is filled with 0.25 atm so3, 0.20 atm so2, and 0.40 atm o2, and allowed to reach equili...
Questions in other subjects:




Mathematics, 16.01.2021 05:40



Mathematics, 16.01.2021 05:40

History, 16.01.2021 05:40


Mathematics, 16.01.2021 05:40


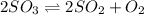
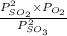
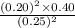
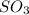 as equilibrium is achieved by using Q, is as follows.
as equilibrium is achieved by using Q, is as follows.



