
Chemistry, 25.12.2019 05:31, kkcheers16
Phosphorous acid, h 3 po 3 ( aq ) , is a diprotic oxyacid that is an important compound in industry and agriculture. p k a1 p k a2 1.30 6.70 calculate the ph for each of the points in the titration of 50.0 ml of 1.8 m h 3 po 3 ( aq ) with 1.8 m koh ( aq ) .

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 13:00, wbrandi118
Using the thermodynamic information in the aleks data tab, calculate the standard reaction free energy of the following chemical reaction: →+p4o10s6h2ol4h3po4s round your answer to zero decimal places.
Answers: 3


Do you know the correct answer?
Phosphorous acid, h 3 po 3 ( aq ) , is a diprotic oxyacid that is an important compound in industry...
Questions in other subjects:

English, 21.08.2020 04:01

Mathematics, 21.08.2020 04:01

Mathematics, 21.08.2020 04:01



English, 21.08.2020 04:01




Mathematics, 21.08.2020 04:01


 and
and 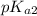 to estimate the values of
to estimate the values of 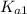 and
and 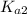 . Therefore:
. Therefore: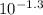 = 0.0501
= 0.0501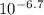 = 1.995*10^(-7)
= 1.995*10^(-7)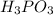 ⇒
⇒ 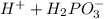
![\frac{[H^{+}]*[H_{2}PO^{-} _{3}]}{[H_{3}PO_{3}]}](/tpl/images/0432/6059/0b4a1.png)
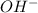 is two times the moles of
is two times the moles of 



