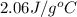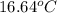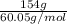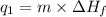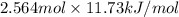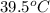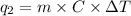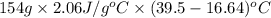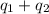Chemistry, 25.12.2019 03:31, rubianny03
Calculate the amount of heat needed to melt 154. g of solid acetic acid (hch3co2) and bring it to a temperature of 39.5 . be sure your answer has a unit symbol and the correct number of significant digits.

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 05:30, alaynagrace1111
What is the mass of each element in a 324.8 sample of co2
Answers: 1

Chemistry, 22.06.2019 18:30, tanviknawale
Which sample at stp has the same number of atoms as 18 liters of ne at stp
Answers: 1

Chemistry, 22.06.2019 19:20, choiboiqg5755
The equation picture below shows which type of nuclear reaction u 235 + n x e 134 + sr 100 + 2n
Answers: 1
Do you know the correct answer?
Calculate the amount of heat needed to melt 154. g of solid acetic acid (hch3co2) and bring it to a...
Questions in other subjects:

Social Studies, 01.06.2021 16:30

Mathematics, 01.06.2021 16:30

History, 01.06.2021 16:30


History, 01.06.2021 16:30

Mathematics, 01.06.2021 16:30

Mathematics, 01.06.2021 16:30

Mathematics, 01.06.2021 16:30


Mathematics, 01.06.2021 16:30